Softeners and Hand Builders - Chemical Fabric Finishing Methods In Textile
17:55
0 comments
Finishing is defined as any chemical process other than preparation or the application of color that imparts useful or desired properties to a textile or apparel product. Although it can be performed on fibers or yarns, finishing usually occurs after the fabric or garment has been prepared and dyed, and is often the last step. Finishing processes can affect many qualities of a fabric or garment, including appearance, performance, or serviceability, and hand, through the use of softeners or hand builders.
Anionic Softeners
Anionic softeners have a negative charge as does wet cotton. Anionic softeners can impart pliability without making the fabric too silky or slick. Because of their charge, they must be padded onto fabric. Not extremely durable, these softeners are normally applied for a one-time effect, often before mechanical finishes such as napping, shearing or compaction are introduced.
- Advantages
Anionic softeners are desirable because they provide the right amount of lubrication between a fabric and a mechanical device. Another advantage is that fabric that is finished with anionics doesn’t easily yellow because these softeners show good stability to heat and are compatible with optical brighteners. They also have good rewetting properties so are preferred for fabrics such as towels that must absorb water.
- Disadvantages
Their disadvantage is that, when compared to other softeners, more product must be used. Anionics are hard to exhaust and may be incompatible with baths containing products such as stabilized cationic emulsions, electrolytes, or resins.
Cationic Softeners
Cationic softeners have a positive charge while wet cotton has a negative charge. Due to their opposing natures, cotton has a high affinity for cationic softeners.
- Advantages
This type of softener produces a fluffy and silky and, on most fabrics, and it doesn’t take much to get this effect on cotton because of the ionic charge of these chemicals. Cationics easily exhaust and are compatible with most resin finishes. Cationics are good for fabric sewability and are quite durable with good tear and abrasion resistance.
- Disadvantages
Cationics are not often used at high levels on white because they tend to yellow at higher processing temperatures. They may also affect shade or lightfastness. Also, in some applications, cationics may cause unwanted water repellency. It is important to note that Anionic and Cationic chemistries are incompatible. Strong cationic softeners can complex or bind with anionic dyestuffs, creating a problem with crocking. Also, the shade may not be reproducible.
Nonionic Softeners
Nonionic softeners have a neutral charge. This includes ethylene oxide derivatives, silicones, and polyethylene. Nonionics have no ionic affinity for cotton and must be applied by a pad application. These softeners show excellent resistance to discoloration and heat, give a dry pliable hand, and can be applied by various methods. The polyethylene type of nonionic softeners creates a soft hand and has excellent non-yellowing properties. The silicone gives a slick, lubricated, and silky hand and blooms the shade, making it look richer and more vibrant. They also increase the performance of durable press. Both polyethylene and silicone softeners improve tear and abrasion resistance, providing sewing lubricity, and are somewhat durable.
Hand Builders
Another class of chemistry for finishing is referred to as hand builders. These finishes are applied when garments require fabrics of strength, firmness, and resistance to abrasions. Hand builders make fabrics less pliable, and control curling when fabrics are difficult to sew.
-
Ayakkabılarda doğru numara seçimi sağlık ve kullanım ömrü açısından önem arz eder. Kesirli Ayakkabı Numaraları Ne Anlama Geliyor? 🤔 Bazı a...
-
Rahat bir kullanım için ayağın genişliği ve uzunluğuna uygun ayakkabıyı seçmek son derece önemlidir. Ayakkabı Genişlik Terimleri: E, F, FX,...
-
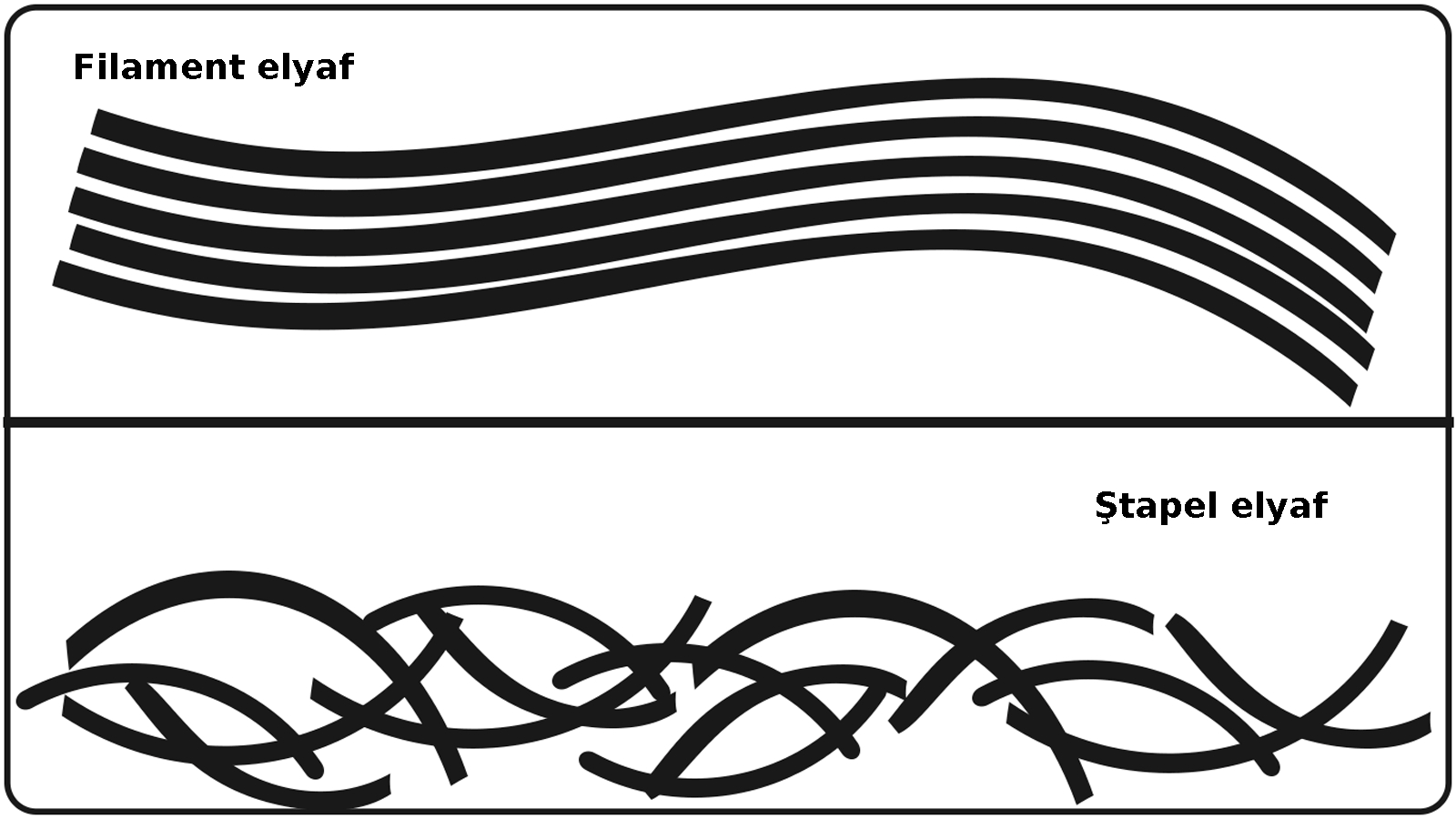
Lif kısaltmaları tekstilde elbise üretiminin her aşamasında kullanılır. Tekstil, Kumaş, Lif ve Elyaf Kısaltmaları : Tekstil endüstrisi, lif...
-
Mavi polycotton nevresim takımı. Polycotton , polyester ile pamuğu (cotton) karıştırarak elde edilen, her iki elyafın en iyi performans ...
-
Kumaşın ön yüzünün ve arka yüzünün gösterimi. Kumaş yüzü (Alm. Stoffvorderseite, Fr. front de tissue, İng. fabric face; face of fab...
-
Elastomultiester lif yapısı. Elastomultiester , iki veya daha farklı kimyasal doğrusal makromoleküllerin iki veya daha farklı aşamada etkil...
-
İş sağlığı ve güvenliği için bazı işletmelerde pr ayakkabı kullanımı gereklidir. Ayakkabılarda rastladığımız "PR" terimi, İngiliz...
-
Türk tekstil ve hazır giyim sektörü: yerli markaların yükselişi. Türkiye'nin lokomotif sektörlerinden biri olan tekstil ve hazır giyim...
-
İngilizce renkler. İngilizcede renk kelimesi Amerikan İngilizcesinde "color", İngilizce İngilizcesinde "colour" olarak ...
-
Yeşil renk ve tonları, sarı ile mavi ışığın birleşmesi sonucu oluşur ve fotosentetik pigmentler nedeniyle bitki yapraklarında yaygın olarak ...
-
Türk tekstil ve hazır giyim sektörü: yerli markaların yükselişi. Türkiye'nin lokomotif sektörlerinden biri olan tekstil ve hazır giyim...
-
Akrilik elyaf, iyi yalıtım özelliğine sahip olmasıyla öne çıkan sentetik bir lif türüdür. Akrilik Elyaf: Tanım ve Özellikler Akrilik, ( Alm....
-
Kumaş numunesi. 1) Yapılarına göre (nasıl yapıldıysa o ismi alır) a) Dokunmamış kumaşlar - Nonwoven , keçeler, kağıt telalar, elyaf, vi...
-
Ünlü Türk modacı ve tasarımcılarının kreasyonları artık dünya moda başkentlerinde sergileniyor. Türkiye'de tekstil ve moda sektörünü...
-
Farklı renk ve türdeki kumaş çeşitleri. Kumaş, ipliklerin, çeşitli yöntemlerle bir araya getirilerek oluşturduğu kaplayıcı yüzeylerd...
-
Türk ayakkabı markaları, yerli ham maddeyi mükemmel işçilik ve estetik tasarımlarla birleştiriyor. Türk malı ayakkabı ürünler, kalitesi ve e...
-
Dünyanın en meşhur modacıları. Dünyaca ünlü modacılar Her sezon önce podyumları sonra da vitrinleri süsleyen özel koleksiyonların arkas...
-
Lif kısaltmaları tekstilde elbise üretiminin her aşamasında kullanılır. Tekstil, Kumaş, Lif ve Elyaf Kısaltmaları : Tekstil endüstrisi, lif...
-
Ayakkabılarda doğru numara seçimi sağlık ve kullanım ömrü açısından önem arz eder. Kesirli Ayakkabı Numaraları Ne Anlama Geliyor? 🤔 Bazı a...
-
Tekstil ürünlerinin etiketlerinde yıkama, kurutma ve ütüleme ile ilgili semboller bulunur. Tekstil Ürünleri için Tavsiye Edilen Yıkama Tali...

















































































































0 yorum:
Yorum Gönder
Merhaba, daha kaliteli bir site için yorumlarınızı bekliyoruz.